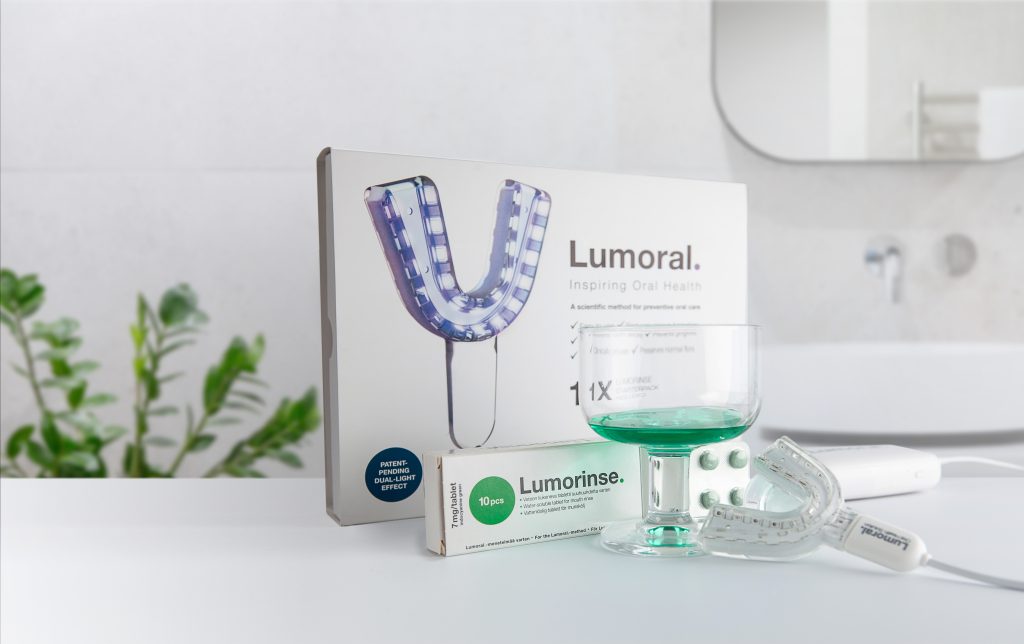
Koite Health Teams Up with 8 Media Ventures to Accelerate Growth in the Nordic Markets
Koite Health, an innovator in the oral health tech space, is thrilled to announce its collaboration with 8 Media Ventures, a leading Nordic media-for-equity company.